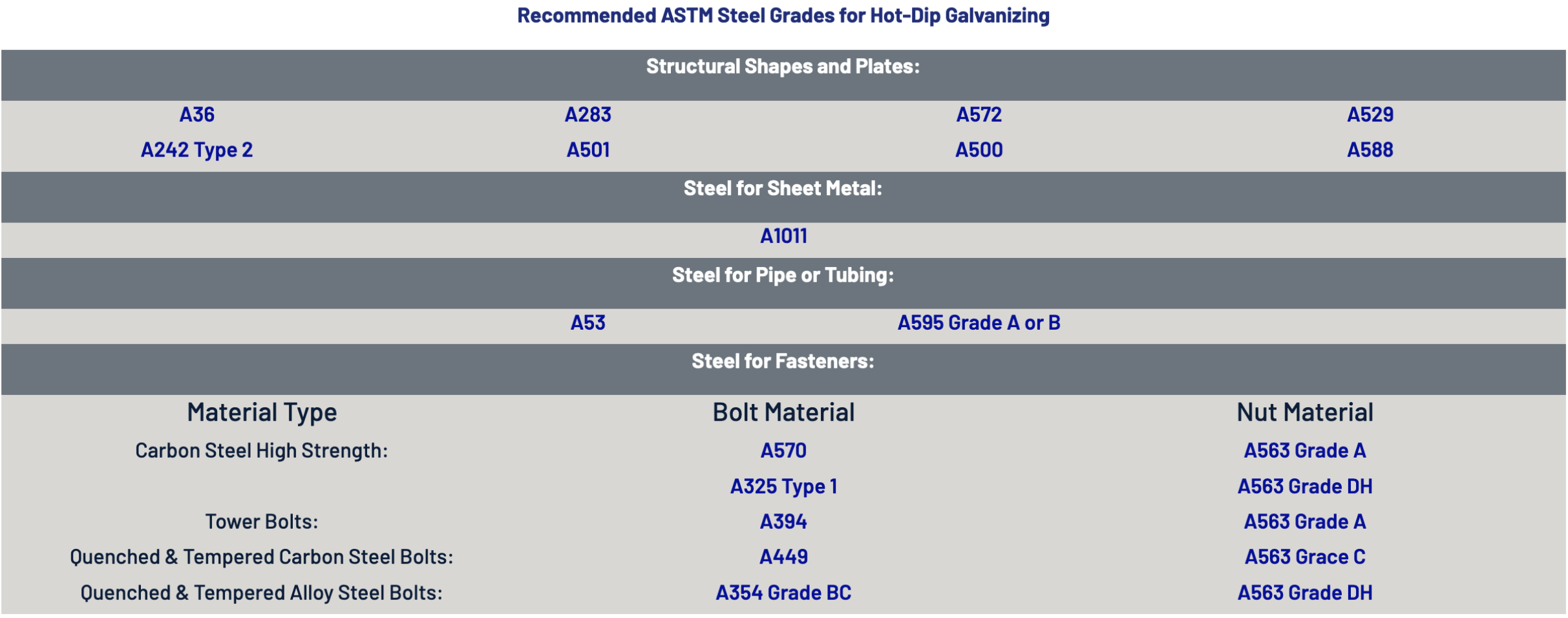
Steel Composition
The chemical composition of the steel is very important to the quality of the galvanized coating since a metallurgical reaction between the zinc and the iron is occurring.
As an example, silicon contents in the steel between 0.05% – 0.11% and above 0.25% will cause the zinc–iron alloy layers to grow abnormally fast. In fact, the silicon causes the galvanizing reaction to continue unabated and the outermost pure zinc layer is eventually consumed in the process. This not only causes the coating to be thicker than normal, but the coating will also most likely consist entirely of alloy layers. The increased presence of these alloy layers in the resulting coating are often brittle and appear as a dark gray, matte finish.
Steel compositions that affect the coating formation and properties in this manner are referred to as “reactive steels”.
General Considerations
The generic composition of a steel to be galvanized that will produce an acceptable coating will be carbon less than 0.25%, phosphorous less than 0.05% and manganese less than 1.3%. Also, as discussed above, in order to avoid the adverse effects of silicon on the galvanized coating, silicon content in the steel should be in the range of 0-0.04% or 0.15-0.25%. Steel with these recommended levels should develop a normal galvanized coating. However, should the composition of these elements fall outside any of the previously stated ranges, the steel will most likely still able to be galvanized provided that the Galvanizer is notified in advance of any deviations.