Coating Characteristics
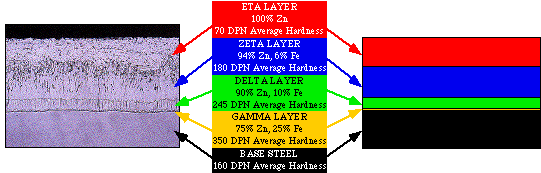

The above photomicrograph and accompanying figure represent a typical cross section of a hot-dip galvanized coating. The outermost (Eta) layer of the coating is formed as the material is withdrawn from the molten zinc bath. This layer is a covering of pure zinc. The underlying series of zinc–iron alloys in the coating are the result of a metallurgical reaction between molten zinc and the steel or iron material being galvanized. As the zinc–iron alloys form, they will grow perpendicularly to the steel surface. The effect this has on corners and edges of material is that the coating there is generally thicker than the surrounding coating, as seen in the micrograph below. This is in sharp contrast to other types of protective coatings that tend to thin out at the edges and corners of material.
The galvanized coating itself is considered to be a self inspecting system. This is because the reaction between the molten zinc and the steel will not occur unless the steel surface is chemically clean. In effect, a galvanized coating that appears sound and continuous is in fact sound and continuous. If a coating should become damaged however, the zinc will continue to provide cathodic protection to the exposed steel. Even if areas as much as 1/4 inch in length and/or width become exposed, the surrounding zinc will provide cathodic protection to this area as long as the coating lasts.
As for mechanical protection, the galvanized zinc coating literally becomes part of the steel substrate thus, an adhesion bond on the order of several thousand psi exists between the two. Also, since the zinc–iron alloys are harder than the underlying steel, they will provide excellent abrasion resistance to the galvanized article. The Eta layer is relatively ductile and also contributes to the protection of the galvanized material by providing good impact resistance. The combination of all these layers to produce the galvanized coating provides toughness and resistance to mechanical damage in transport, erection and service.
The thickness and appearance of the galvanized coating are affected by several factors. These include:
The chemical composition of the steel
The surface condition of the steel In general, rough steel surfaces will cause the galvanizing reaction to produce thicker coatings due to the increased surface area. These thicker coatings, however, will be rough and have a generally poor appearance.
Bath immersion time Galvanizing is a diffusion process. As with all diffusion processes, the reaction between the molten zinc and the steel or iron will proceed quickly at first but will slow down as the alloy layers grow and become thicker. Thus, continued immersion or dipping material more than once will not produce a significantly thicker coating except in the case of reactive steels.
Bath withdrawal rate The withdrawal rate of material from the galvanizing bath has the greatest effect on the outermost layer of pure zinc. A rapid withdrawal will produce the thickest coating because the largest amount of zinc will be carried out on the material and will subsequently solidify and become the pure zinc layer. Slower withdrawal allows the zinc to effectively drain back into the kettle producing smoother, thinner, and more uniform coatings.
Steel cooling rate Slower cooling rates, such as those experienced with air cooling or even with thicker sections that have been water quenched, allow the zinc/iron alloying reaction to continue. The inner alloy layers will use the outer Eta layer for their supply of zinc causing the coating to become dull, matte gray. Quickly reducing the temperature to less than 300 degrees Fahrenheit will halt the galvanizing reaction and minimize the formation of dull or matte surfaces.
